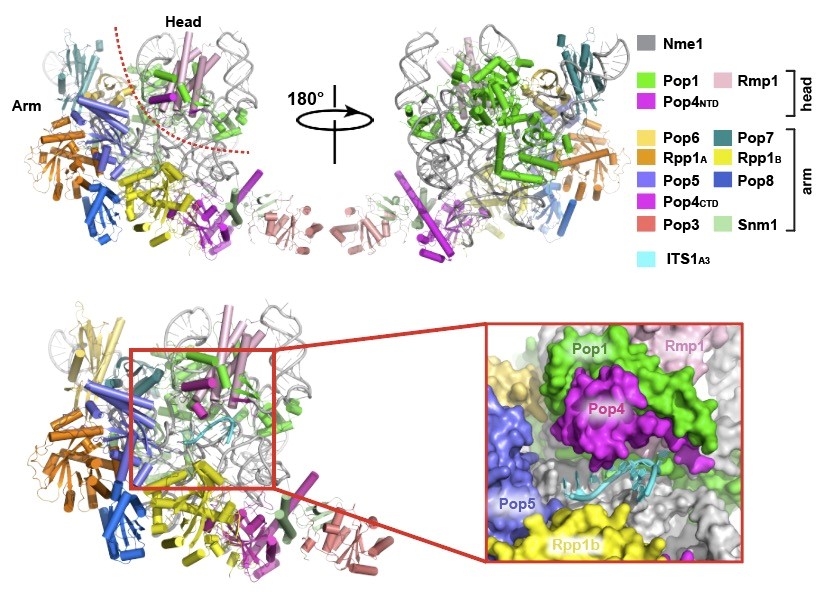
The research team directed by Prof. Lei Ming (雷鸣) at Shanghai Institute of Precision Medicine, the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine recently reported the structural insights into precursor rRNA processing mechanism by eukaryotic RNase MRP, which was published as research article in Science (2018, Vol. 362, Issue 6415, eaat 6678) Ribonuclease (RNase) MRP is a conserved eukaryotic ribonucleoprotein complex that plays essential roles in pre-ribosomal RNA (pre-rRNA) processing and cell cycle regulation. In contrast to RNase P, which selectively cleaves tRNA-like substrates, it has remained a mystery how RNase MRP recognizes its diverse substrates. Prof. Lei’s group determined the cryo-EM structures of Saccharomyces cerevisiae RNase MRP holoenzymes alone and in complex with in complex with an ITS1 fragment containing the A3 cleavage site of the pre-rRNA. These structures reveal that, although the catalytic center of RNase MRP is nearly identical to that in RNase P, a striking local refolding of key protein subunits transforms RNase MRP into a distinct ribonuclease to process single-stranded RNAs by recognizing a short consensus sequence. The RNase MRP-ITS1A3 complex structure demonstrates that RNase MRP recognizes a short single-stranded region of ITS1A3 and two nucleotides C2 and C4 make sequence specific interactions with RNase MRP. Combination of the structural and biochemical data, we defined a short consensus sequence for RNase MRP substrate as 5′-*RCRC-3′ (*: cleavage site). The configuration of this RNA-based catalytic center of RNase MRP is identical to that in the RNase P-pre-tRNA complex, suggesting that although the biochemistry and cryo-EM structure of the RNase MRP-ITS1A3 complex reveal clear substrate recognition differences compared to RNase P-pre-tRNA interactions, both essential RNase P and MRP ribonucleopro-tein complexes appear to share a common two metal-ion SN2-type catalytic mechanism. The structures also provide an evolutionary model depicting how such a simple ribozyme evolved into a much more complex protein components in higher organisms. This study represents a major step forward for mechanistic understanding of the function of eukaryotic RNase MRP and provides evolutionary insights into the essential ribozyme of RNase MRP.
Cryo-EM structures of human RNase MRP holoenzyme and in complex with ITS1A3 of pre-rRNA.
|