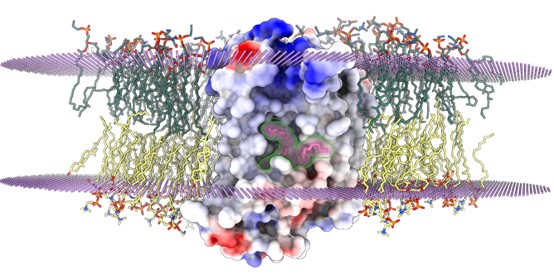
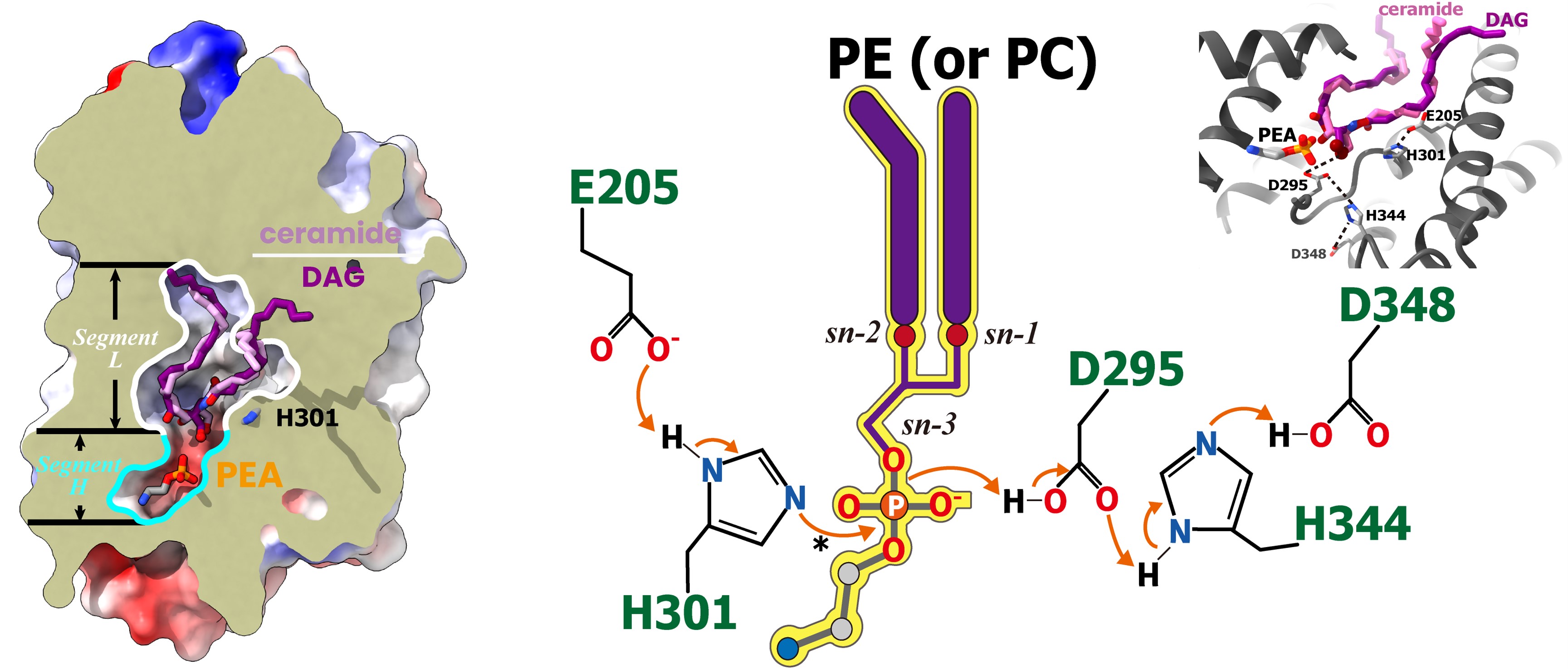
Sphingomyelin (SM) is a vital component of biological membranes, playing crucial roles in regulating membrane properties and serving as a reservoir for bioactive molecules. The biosynthesis of sphingomyelin is orchestrated by the sphingomyelin synthase (SMS) family, which includes SMS1, SMS2, and SMSr. SMS1 and SMS2 are known to possess sphingomyelin synthase activity, while SMSr exhibits ceramide phosphoethanolamine (CPE) synthase activity. Understanding the structural and mechanistic intricacies of these enzymes is fundamental to elucidating sphingolipid metabolism and its implications in cellular physiology and pathology. On February 22, 2024, Prof. CAO Yu's group at SHIPM published a research article in Nature Structural & Molecular Biology entitled "Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis". In this study, CAO Lab present the cryo-electron microscopic structures of human SMSr in complexes with ceramide, diacylglycerol/phosphoethanolamine, and ceramide/phosphoethanolamine. These structures unveil a hexameric arrangement of the enzyme, with a central reaction chamber nestled between its transmembrane helices. Within this architectural framework, researchers identify a catalytic pentad, E-H/D-H-D, strategically positioned at the interface of the lipophilic and hydrophilic segments of the reaction chamber. This configuration suggests a role for these residues in orchestrating the enzymatic catalysis of sphingolipid biosynthesis. Moreover, these findings shed light on the intricate two-step synthesis process catalyzed by SMSr. This process involves the hydrolysis of phosphoethanolamine from phosphatidylethanolamine (PE-PLC hydrolysis) followed by the transfer of the phosphoethanolamine moiety to ceramide, resulting in the formation of ceramide phosphoethanolamine. Unraveling these mechanistic details not only deepens our understanding of the catalytic mechanism of SMSr but also broadens our insights into the broader landscape of sphingolipid metabolism. By elucidating the structural architecture and mechanistic intricacies of human SMSr, CAO lab’s study contributes significantly to the burgeoning field of lipid biochemistry. These insights pave the way for the development of targeted therapeutic strategies aimed at modulating sphingolipid metabolism for the treatment of various diseases, including cancer, neurodegenerative disorders, and metabolic syndromes. Overall, their research provides a foundation for further exploration into the role of sphingolipids in cellular homeostasis and disease pathogenesis. The article is available via URL: https://www.nature.com/articles/s41594-024-01237-2 |