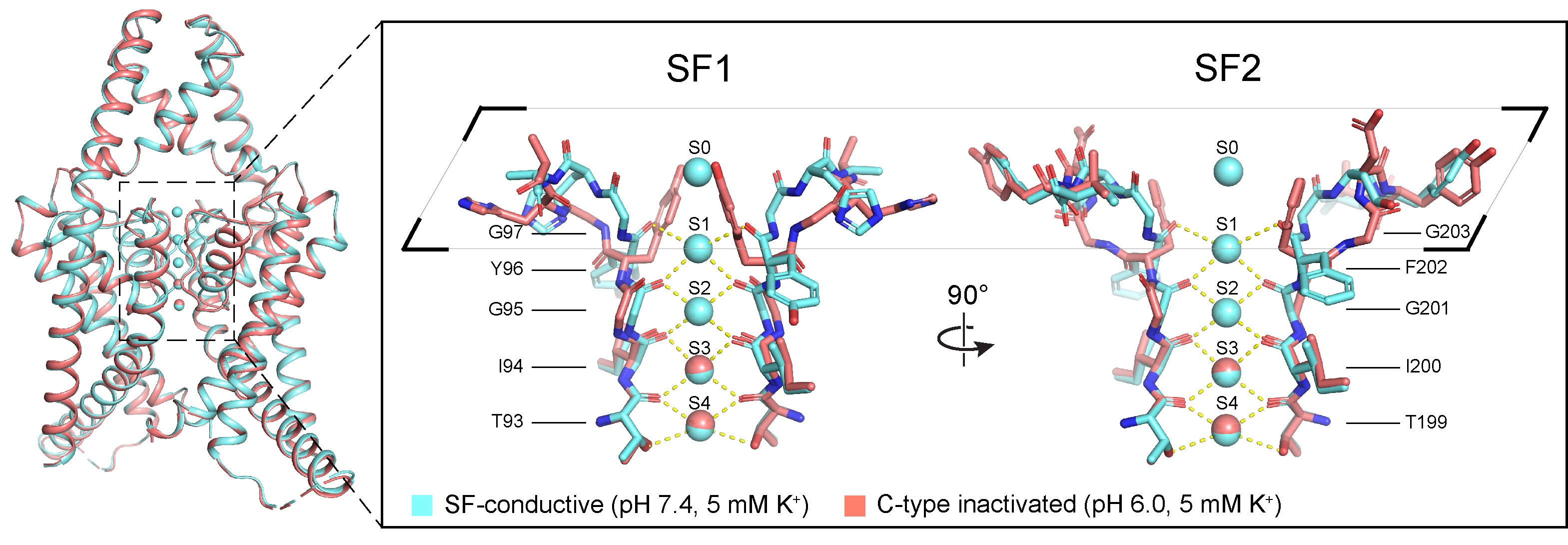
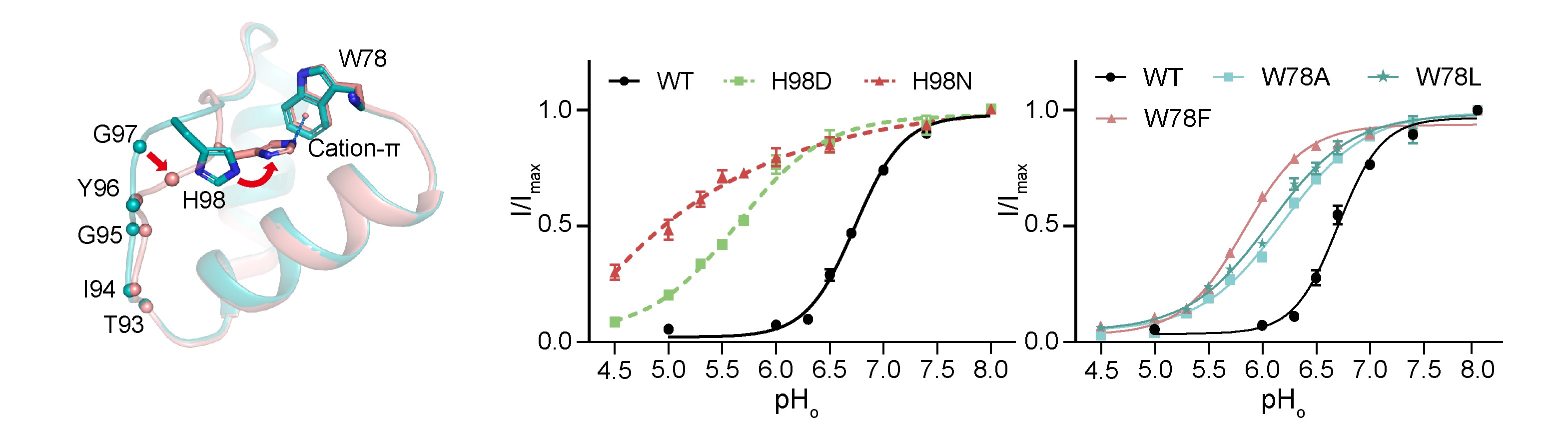
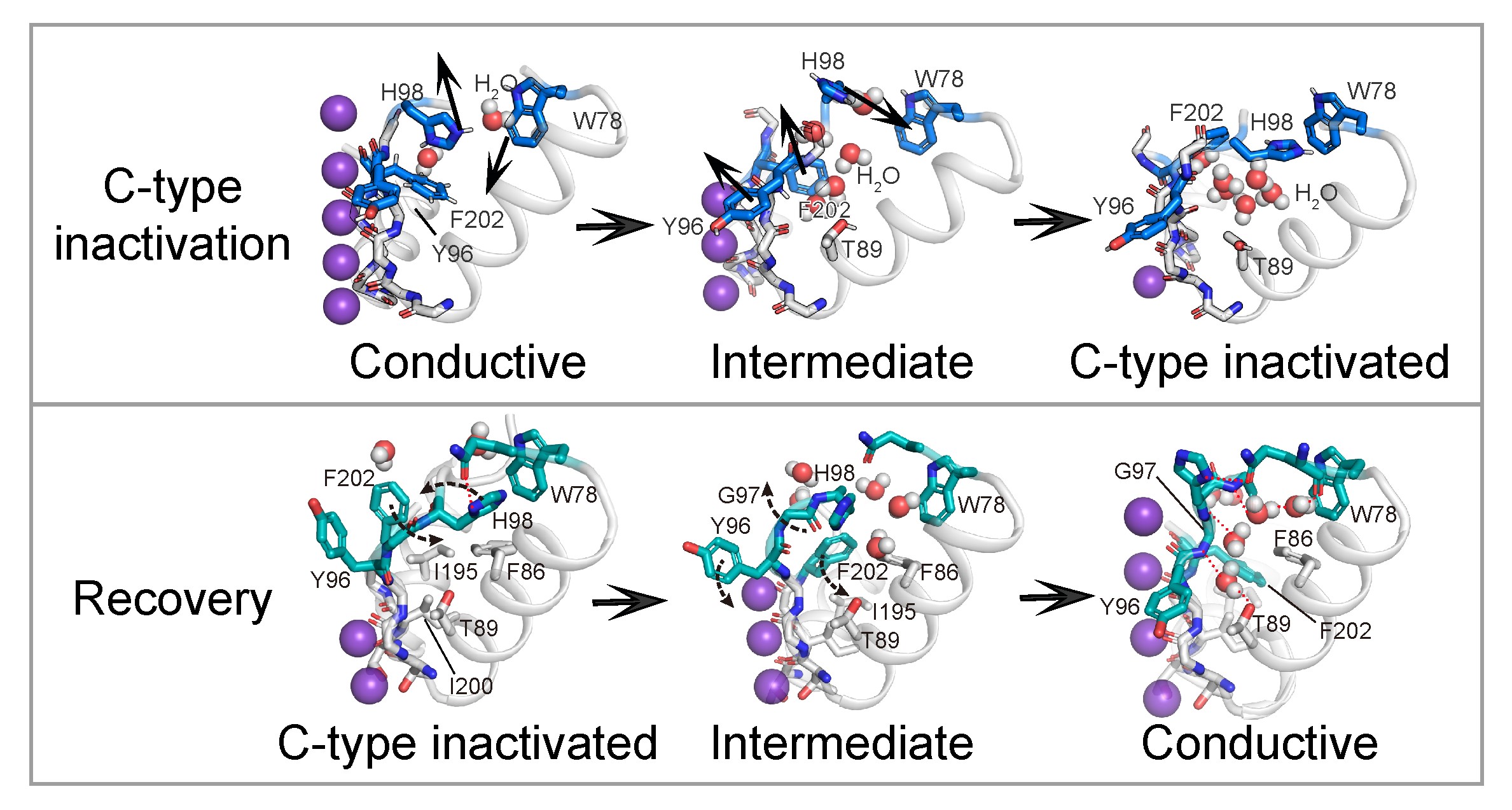
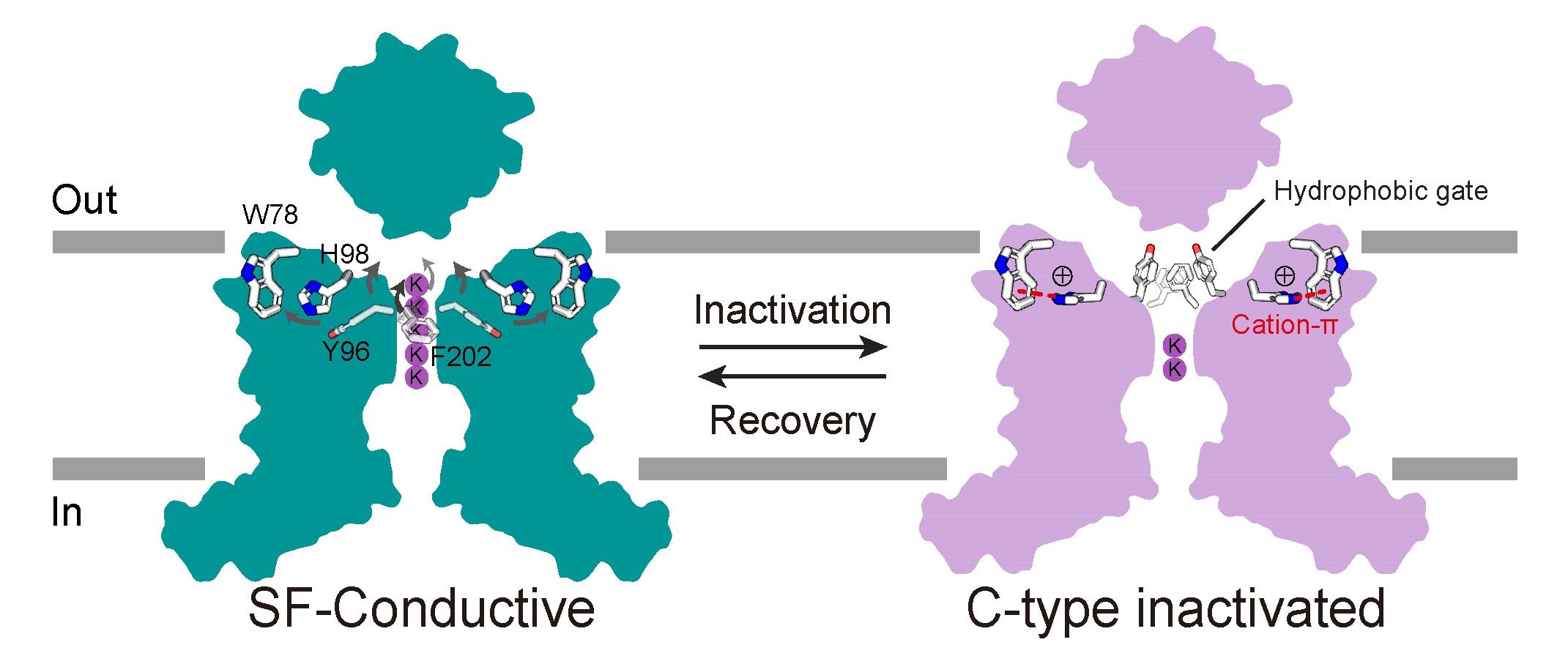
Potassium channels regulate ion homeostasis and play essential roles in the electrical pulses of excitable systems such as the nervous, cardiovascular and endocrinal systems. The structural basis and molecular mechanisms by which environmental stimuli act on different potassium channels to modulate their ion permeability are classical and important scientific questions. The members of the potassium channel superfamily are diverse, and their gating mechanisms are broadly categorized into two types. Whereas one type is formed at the transmembrane helical bundle crossings on the inner side of the membrane, the other is underpinned by the selective filter close to the outer side of the cell, whose conformation dynamics affect potassium ion permeability through a mechanism known as C-type gating. The structural basis of the C-type gating has been extensively studied. It is generally accepted that the C-type inactivation results from localized small-scale structural rearrangements of the selectivity filter that impar potassium ion coordination. However, how such rearrangements are coupled to environmental stimuli is not fully understood. On April 17th, 2024, Dr. Shanshuang Chen’s group from Shanghai Institute of Precision Medicine, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine and Prof. Huaiyu Yang’s group from School of Life Sciences, East China Normal University, Shanghai, published a research article in PNAS entitled “C-type Inactivation and Proton Modulation Mechanisms of the TASK3 Channel” in collaboration. In the central and peripheral nervous system, TASK3 is involved in the regulation of resting membrane potential by mediating constitutive potassium efflux, and has shown promising potential as an analgesic target. Since TASK3 lacks the transmembrane helical bundle crossing and can be inhibited by extracellular protons, we chose it as a model to study its C-type gating and proton modulation. The team determined the cryo-electron microscopic structure of human TASK3 under neutral and acidic conditions, and captured the selective filter at the conductive and C-type inactivated conformation, respectively (Figure 1). Figure 1. The conductive and C-type inactivated conformations of the selectivity filter. Comparison of the two conformations revealed that the overall structure of TASK3 largely remained unchanged upon C-type inactivation, but the selectivity filter underwent large-scale rearrangements, which not only resulted in compromised potassium ion coordination at the S1-S2 positions of the selectivity filter, but also in the flip of its mainchain to drive the formation of a hydrophobic gate by Tyr96 and Phe202 at the extracellular exit of the channel, which prevented potassium ion permeation. His98 and Trp78 were far apart from each other under neutral conditions. By contrast, when His98 became protonated upon acidification, it came close to Trp78 to form a cation-π interaction, thereby stabilizing the C-type inactivated conformation. To test this hypothesis, the team employed electrophysiology to show that point mutations of His98 or Trp78 significantly attenuated the response of TASK3 to extracellular acidification, supporting the mechanistic hypothesis that His98 is the proton sensor of TASK3 (Figure 2). Figure 2. Mutagenesis and electrophysiology revealing key residues in TASK3 modulation. Given that His98 acted as the proton sensor of TASK3, the team continued to address if His98 protonation was sufficient to induce C-type inactivation by molecular dynamics simulations (Figure 3). The molecular dynamics simulations showed that whereas deprotonation of His98 stabilized the conductive selectivity filter, simple alteration of His98 into protonated state caused the selectivity filter rearrangements into the C-type inactivated state under the same conditions. Similarly, whereas protonated His98 stabilized the C-type inactivated selectivity filter, the selectivity filter recovered to re-establish the conductive conformation upon deprotonation of His98. Therefore, the above results collectively supported the hypothesis that the C-type gating of TASK3 was coupled to His98 protonation dynamics. Figure 3. C-type inactivation and recovery recapitulated by molecular dynamics simulations. Using TASK3 channels as a model, this study combined structural biology, electrophysiology and molecular dynamics simulations to elucidate the molecular mechanism by which the C-type gating is coupled to environmental stimuli. The C-type gating of TASK3 is underpinned by dynamic structural equilibrium between the canonical conductive and the non-canonical inactivated selectivity filter conformation, and the equilibrium is modulated by the formation of a cation-π interaction between His98 and Trp78 in a His98 protonation-dependent manner. Together, this study provided a framework for future development of small-molecule drugs targeting the dynamic conformation of the K2P channels (Figure 4). Figure 4. The C-type gating mechanism of TASK3 Dr. Huajian Lin, a postdoc researcher from Shanghai Institute of Precision Medicine, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Junnan Li, a graduate student and Dr. Qiansen Zhang, a junior investigator from School of Life Sciences, East China Normal University, Shanghai, are co-first authors of the article. Dr. Shanshuang Chen and Prof. Huaiyu Yang are the co-responding authors of the article. Dr. Shanshuang Chen’s lab is interested in the transmembrane signal transduction in the nervous system. The lab holds openings for postdoc researcher, and motivated researchers are encouraged to apply. The article is available at https://www.pnas.org/doi/10.1073/pnas.2320345121 |