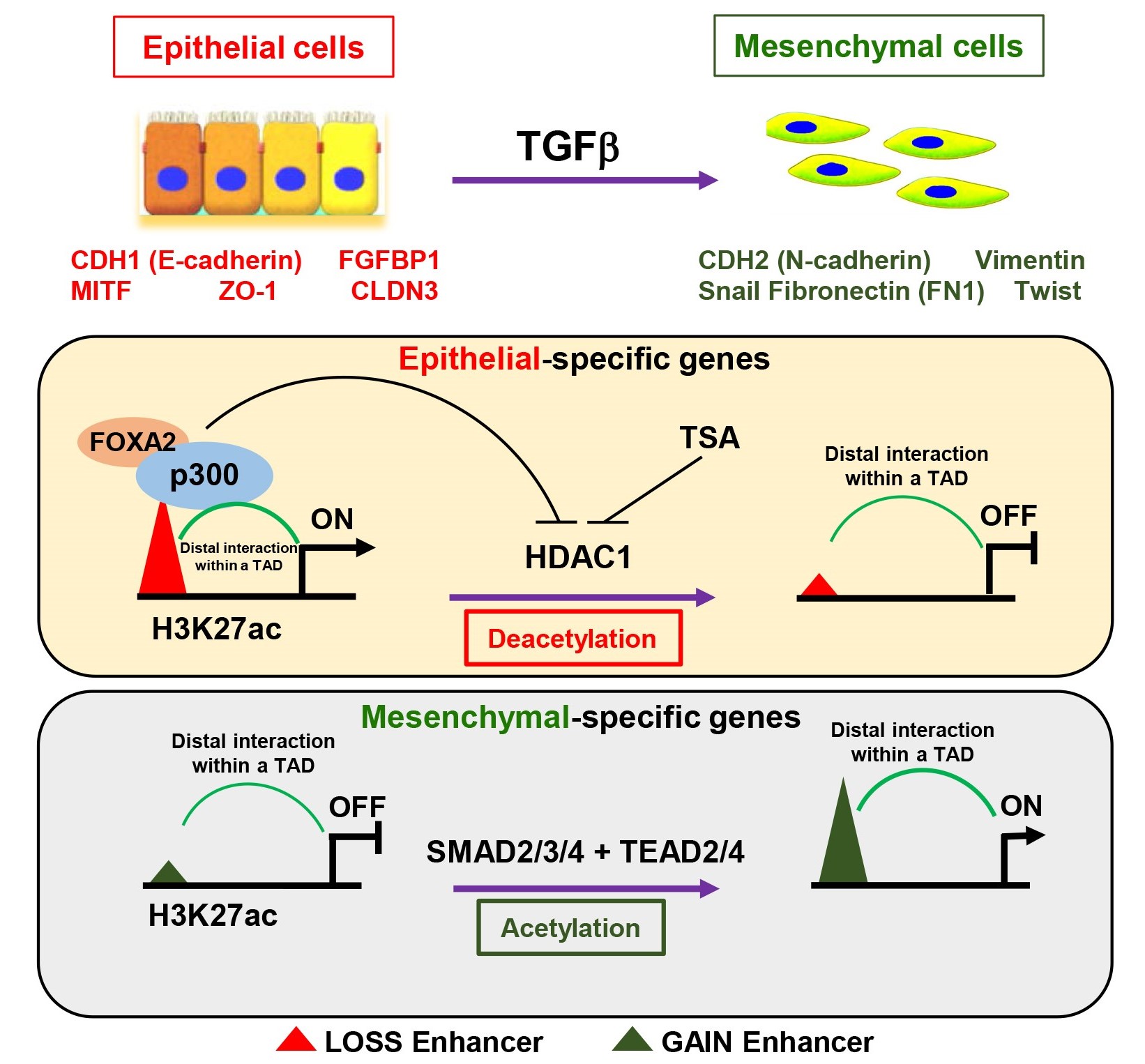
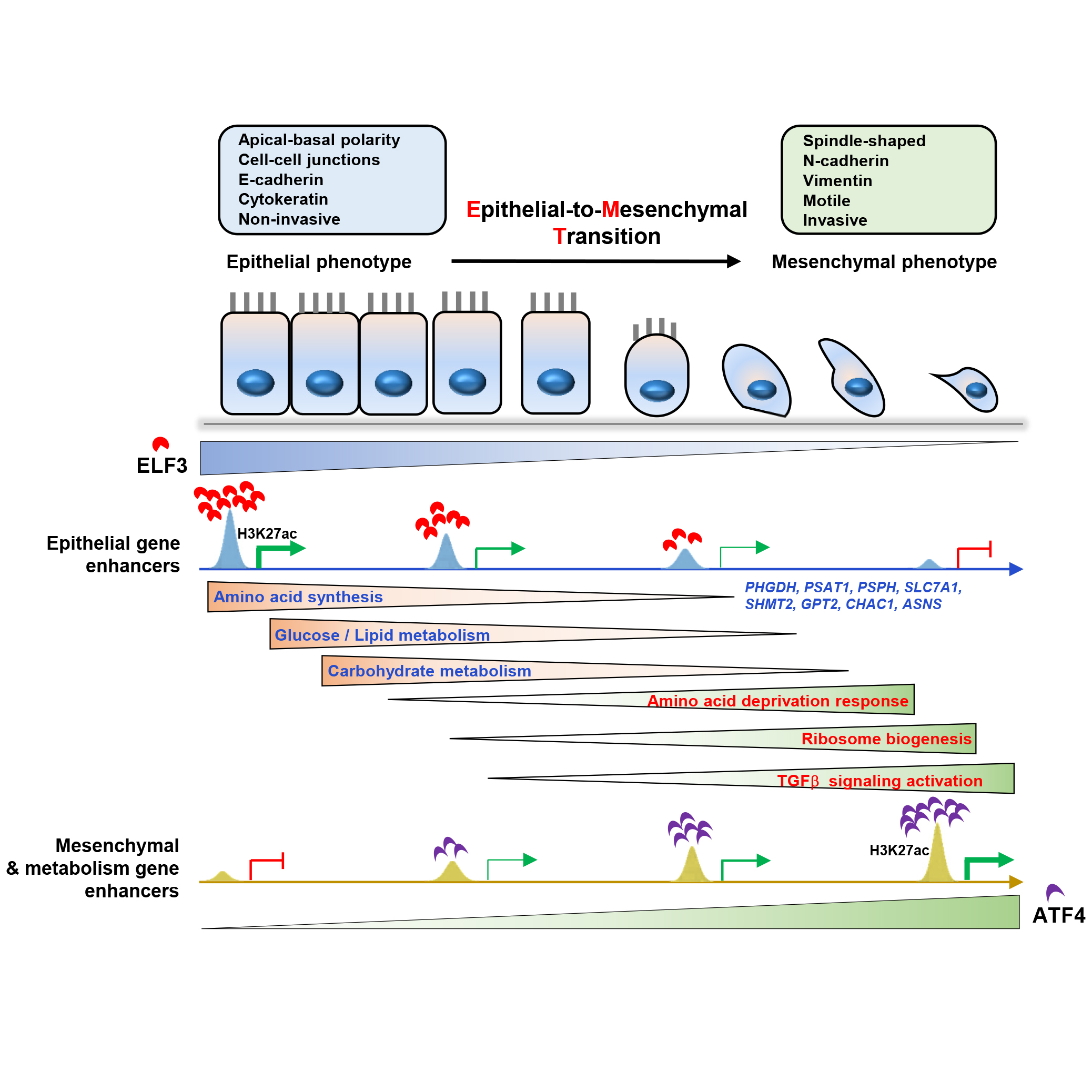
Epithelial mesenchymal transition (EMT) is a complex and finely regulated biological process that occurs during development, regeneration, tumor occurrence, and metastasis. During this process, epithelial cells lose their epithelial cell characteristics, including cell polarity and cell-cell contact inhibition, and obtain a mesenchymal cell phenotype characterized by cell migration and invasion, which is conducive to cell migration behavior and integration into surrounding or distant tissues, especially playing an "accomplice" role in tumor invasion and metastasis. EMT is closely related to carcinogenesis, tumor cell stemness, cancer metastasis, and therapy resistance. During cancer progression, EMT can be induced by hypoxia, cytokines, nutrient deficiency, metabolic changes, immune response, tumor microenvironment, and anti-tumor drugs. Among them, the TGF-beta signaling pathway is an extremely important factor in inducing EMT, and its important function in normal tissue development, regeneration, and other processes makes its function more special in the carcinogenesis, and it is also more likely to become the culprit of cancer metastasis. In our previous research (Molecular Therapy, 2020), Yunbo Qiao 's research group and Guisheng Zhong 's group from ShanghaiTech University analyzed the dynamic changes of enhancers during TGF-beta-induced EMT. They found that the loss of epithelial specific enhancers induced by TGF-beta signal and the activation of mesenchymal enhancers were important reasons for the occurrence of EMT process. Among them, FOXA2/p300 complex is a key regulatory factor for maintaining epithelial enhancer activity, while HDAC1 mediates the H3K27ac deacetylation and enhancer activity loss in epithelial cells. The SMAD2/3 and TEAD2/4 complexes mediate the establishment of mesenchymal enhancers and the activation of mesenchymal genes. At the same time, we also found that the three-dimensional structure of chromatin did not undergo significant changes before and after TGF-beta treatment. Epithelial marker genes and their enhancers, as well as mesenchymal marker genes and their characteristic enhancers, displayed strong interactions in their initial states. We speculate that the pre-folding and chromatin interaction between enhancers and their target genes ensures that cells respond quickly to external signal stimuli. Molecular Therapy, 2020 On April 15, 2024, Dr. Qiao Yunbo's group from Shanghai Institute of Precision Medicine, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, and Ye Bo's team from Shanghai Chest Hospital published a research article entitled "Switch of ELF3 and ATF4 transcriptional axis programs the amino acid infection linked affinity to mesenchymal transition" online in Molecular Therapy. During the TGFbeta-induced EMT, multiple transcription factors are inactivated or activated. Therefore, this study delves into how different transcription factors synergistically regulate gene expression and cellular state changes. This study analyzed the inactivated epithelial enhancers induced by TGF-beta, and found that ELF3 may be an important upstream regulator for maintaining epithelial enhancer activity. Knocking down ELF3 in tumor cells with epithelial features resulted in morphological changes similar to EMT and weak response to TGF-beta stimulation. ELF3-knockdown also resulted in the loss of some epithelial enhancers and activation of mesenchymal enhancers, partially overlapping with the differential enhancers induced by TGF-beta, indicating that ELF3 is indeed a key transcription factor in maintaining epithelial features and enhancer activity. Further study found that knocking down ELF3 leads an EMT-like state similar to amino acid deficiency-induced EMT, accompanied by the downregulation of lipid/carbohydrate metabolism and the upregulation of gene expression related to aminoacyl tRNA biosynthesis pathway. Therefore, it is speculated that the inactivation of ELF3 may lead to a partial EMT status. Analyzing the ELF3 knockdown-inactivated enhancers, it was found that SMAD4, TEAD4, and ATF4 have potential binding sites in these enhancers. Given that the above work has preliminarily elucidated the functions of SMAD4 and TEAD4 in mesenchymal enhancer activation, and ATF4 is also an important transcription factor regulating amino acid metabolism, this study focuses on the synergistic regulatory function of ATF4 and ELF3 in the EMT process. It was found that ELF3 and ATF4 had few enhancers that co-bound with each other, exhibiting mutually exclusive characteristics. We also show that ELF3 knockdown results in the loss of epithelial enhancer activity, while ATF4 binds and activates some mesenchymal enhancers and regulatory factors of amino acid metabolism such as CHAC1 and PHGDH, thereby promoting EMT processes similar to those induced by amino acid deficiency. We speculate that the inactivation of ELF3 leads to the upregulation of ATF4 expression, causing cells to perceive a "pseudo state" similar to amino acid deficiency, resulting in a series of transcriptional cascades similar to amino acid deficiency, including ATF4-induced upregulation of TARS, YARS, EARS, YARS, etc., which in turn leads to EMT-like features in cells and helps them acquire migration ability and promote tumor development. In summary, this work reveals that different transcription factors synergistically regulate enhancer activity and downstream target gene expression, helping tumor cells respond to external conditions and survive under adverse conditions, and obtain EMT features, which are important inducers of cancer metastasis. Molecular Therapy, 2024 Link to the paper: https://www.cell.com/molecular-therapy-family/molecular-therapy/abstract/S1525-0016(24)00242-9 |